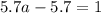Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is a possible formula unit? (2 points) select one: a. pbo b. li2b c. al2pb3 d. clo
Answers: 1
You know the right answer?
For the chemical equation so2(g)+no2(g)↽−−⇀so3(g)+no(g) the equilibrium constant at a certain temper...
Questions

English, 30.03.2020 23:49

Mathematics, 30.03.2020 23:49

Mathematics, 30.03.2020 23:49

History, 30.03.2020 23:49

Biology, 30.03.2020 23:49






Mathematics, 30.03.2020 23:49


Mathematics, 30.03.2020 23:49


Mathematics, 30.03.2020 23:49



Mathematics, 30.03.2020 23:49


Mathematics, 30.03.2020 23:49

![Kc=\frac{[NO][SO_{3}]}{[NO_{2}][SO_{2}]}](/tpl/images/0156/8615/e939f.png)
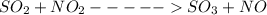
![Kc=3.80 = \frac{[1][1]}{[a-1][1.5]}](/tpl/images/0156/8615/9a395.png)