

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Consider the following equation: n2o4(g) ⇄ 2 no2(g) kc = 5.8 × 10-3 if the initial concentration of...
Questions


Geography, 03.12.2020 01:30

Business, 03.12.2020 01:30

Mathematics, 03.12.2020 01:30



English, 03.12.2020 01:30

Mathematics, 03.12.2020 01:30

Geography, 03.12.2020 01:30


Mathematics, 03.12.2020 01:30



Biology, 03.12.2020 01:30

Advanced Placement (AP), 03.12.2020 01:30

Mathematics, 03.12.2020 01:30


Mathematics, 03.12.2020 01:30


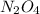 at equilibrium is, 0.03977 M
at equilibrium is, 0.03977 M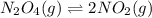
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0156/8648/271f5.png)
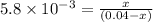
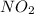 at equilibrium = 2x M = 2 × 0.00023 = 0.00046 M
at equilibrium = 2x M = 2 × 0.00023 = 0.00046 M

