
Chemistry, 01.08.2019 04:10 ramirezdolores
Determine the rate law and the value of k for the following reaction using the data provided. co(g) cl2(g) → cocl2(g)[co]i (m)[cl2]i(m)initial rate (m-1s-1)0.250.400.6960.250.801.970. 500.803.94a) rate

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2
You know the right answer?
Determine the rate law and the value of k for the following reaction using the data provided. co(g)...
Questions

English, 06.07.2019 10:30



Social Studies, 06.07.2019 10:30

Mathematics, 06.07.2019 10:30



Mathematics, 06.07.2019 10:30





Chemistry, 06.07.2019 10:30




Mathematics, 06.07.2019 10:30

World Languages, 06.07.2019 10:30


Biology, 06.07.2019 10:30

![\text{Rate}=k[CO]^1[Cl_2]^{\frac{3}{2}}](/tpl/images/0156/8683/d3cd0.png) and value of 'k' is
and value of 'k' is 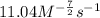
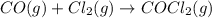
![\text{Rate}=k[CO]^a[Cl_2]^b](/tpl/images/0156/8683/d760d.png)
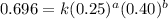 ....(1)
....(1)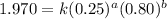 ....(2)
....(2)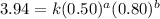 ....(3)
....(3)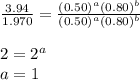
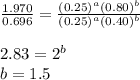
![0.696=k[0.25]^1[0.40]^{\frac{3}{2}}\\\\k=11.04M^{-\frac{7}{2}}s^{-1}](/tpl/images/0156/8683/569d9.png)


