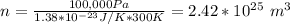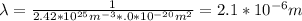Chemistry, 01.08.2019 04:30 ddatsman1730
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the temperature is 300 k given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? boltzmann's constant is k = 1.38 × 10-23 j/k.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the te...
Questions


History, 16.12.2019 07:31


History, 16.12.2019 07:31


Biology, 16.12.2019 07:31

Mathematics, 16.12.2019 07:31

Spanish, 16.12.2019 07:31


Mathematics, 16.12.2019 07:31



Computers and Technology, 16.12.2019 07:31



Mathematics, 16.12.2019 07:31

History, 16.12.2019 07:31

Mathematics, 16.12.2019 07:31

World Languages, 16.12.2019 07:31

Health, 16.12.2019 07:31