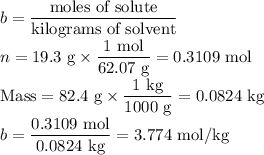
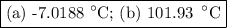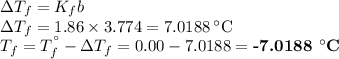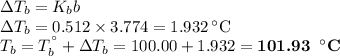An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a)...

Chemistry, 01.08.2019 05:10 pricillagarcia2002
An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a) compute the freezing point of the solution. (assume a density of 1.00 g/ml for water.)
(b)compute the boiling point of the solution. (assume a density of 1.00 g/ml for water.)
express your answer using five significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
Questions

Mathematics, 07.01.2021 19:00

Mathematics, 07.01.2021 19:00


Physics, 07.01.2021 19:00

Mathematics, 07.01.2021 19:00

Arts, 07.01.2021 19:00




Mathematics, 07.01.2021 19:00


English, 07.01.2021 19:00

Social Studies, 07.01.2021 19:00

Chemistry, 07.01.2021 19:00




Mathematics, 07.01.2021 19:00


Mathematics, 07.01.2021 19:00