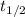Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
The decomposition of nitrogen dioxide to nitrogen monoxide and oxygen gas is a second order process...
Questions

History, 12.10.2020 02:01


History, 12.10.2020 02:01



Mathematics, 12.10.2020 02:01




Health, 12.10.2020 02:01



Mathematics, 12.10.2020 02:01


Physics, 12.10.2020 02:01

History, 12.10.2020 02:01


Mathematics, 12.10.2020 02:01

English, 12.10.2020 02:01

Mathematics, 12.10.2020 02:01

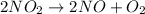
![t_{1/2} = \frac{1}{[A_{0}]k}](/tpl/images/0157/0306/7be8e.png) ....... (1)
....... (1) . So, expression for this will be as follows.
. So, expression for this will be as follows.![\frac{1}{k} [\frac{1}{[A]_{f}} - \frac{1}{[A_{0}]}]](/tpl/images/0157/0306/b672b.png) ...(2)
...(2)![[A]_{f}](/tpl/images/0157/0306/aeb4f.png) is the final concentration that is,
is the final concentration that is, ![\frac{[A]_{0}}{4}](/tpl/images/0157/0306/009c9.png) here and
here and ![[A]_{i}](/tpl/images/0157/0306/49e7e.png) is the initial concentration.
is the initial concentration.![\frac{1}{k} [\frac{4}{[A]_{0}} - \frac{1}{[A_{0}]}]](/tpl/images/0157/0306/9d389.png)
![\frac{3}{[A_{0}]k}](/tpl/images/0157/0306/df0b5.png) ...... (3)
...... (3)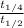 =
= ![\frac{3}{[A_{0}]k} \times [A_{0}]k](/tpl/images/0157/0306/a9d9d.png)