
Chemistry, 01.08.2019 05:20 idontcare2003
Suppose that 25.0 ml of 0.10 m ch3cooh (aq) is titrated with 0.10 m naoh (aq). what is the ph after the addition of 10.0 ml of 0.10 m naoh (aq)? (notice that the total volume of the solution changes with the addition of 10.0 ml of 0.10 m naoh)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
Suppose that 25.0 ml of 0.10 m ch3cooh (aq) is titrated with 0.10 m naoh (aq). what is the ph after...
Questions

Arts, 30.01.2020 16:43


Chemistry, 30.01.2020 16:43



Mathematics, 30.01.2020 16:44

Mathematics, 30.01.2020 16:44

Mathematics, 30.01.2020 16:44

Mathematics, 30.01.2020 16:44


English, 30.01.2020 16:44

Chemistry, 30.01.2020 16:44


Geography, 30.01.2020 16:44

Mathematics, 30.01.2020 16:44

Mathematics, 30.01.2020 16:44


Mathematics, 30.01.2020 16:44


Physics, 30.01.2020 16:44

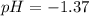
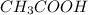 is titrated with 0.10 M NaOH(aq).
is titrated with 0.10 M NaOH(aq).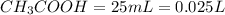
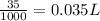
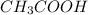 =0.10 M
=0.10 M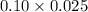 =0.0025 moles
=0.0025 moles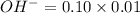 =0.001mole
=0.001mole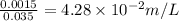
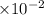 ]=-log4.28+2 log 10=-0.631+2
]=-log4.28+2 log 10=-0.631+2

