

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

You know the right answer?
Agaseous hydrogen and carbon containing compound is decomposed and formed to contain 82.66% carbon a...
Questions


Mathematics, 30.11.2019 16:31



Physics, 30.11.2019 16:31

English, 30.11.2019 16:31

Mathematics, 30.11.2019 16:31











Biology, 30.11.2019 16:31

Biology, 30.11.2019 16:31

History, 30.11.2019 16:31

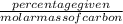
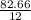
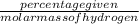
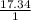
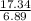 = 2.5
= 2.5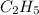 .
.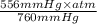
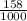 equals 0.158 L.
equals 0.158 L.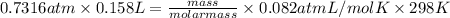
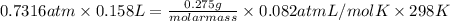
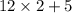 = 29.
= 29.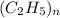 = 58
= 58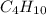 .
.

