
Chemistry, 02.08.2019 20:30 LeoValdez5782
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, so its concentration is so low that we can treat it as an ideal solute. 1) suppose that at 283 k, the equilibrium concentration is 9 × 10-4 water molecules per oil molecule, and it takes 2.208 × 10-20 j to transfer one water molecule into the oil. what is the equilibrium concentration at 293 k, assuming that nothing else changes

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, s...
Questions


Mathematics, 01.08.2019 02:00


English, 01.08.2019 02:00

English, 01.08.2019 02:00


Biology, 01.08.2019 02:00

English, 01.08.2019 02:00


Mathematics, 01.08.2019 02:00


English, 01.08.2019 02:00

Biology, 01.08.2019 02:00

Physics, 01.08.2019 02:00



Mathematics, 01.08.2019 02:00

Chemistry, 01.08.2019 02:00

Mathematics, 01.08.2019 02:00

English, 01.08.2019 02:00

 J/molecule
J/molecule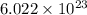 atoms.
atoms.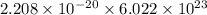 J/mol
J/mol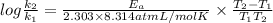
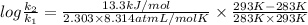
 = 0.08377
= 0.08377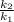 = 1.213 =
= 1.213 = 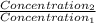
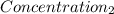 =
= 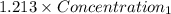
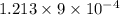
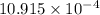 water molecules per oil molecule
water molecules per oil molecule

