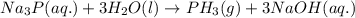Chemistry, 02.08.2019 20:40 malikxyo224
When lithium nitride, li3n(s) , is treated with water, h2o(l) , ammonia, nh3(g) , is produced. predict the formula of the gas produced when sodium phosphide, na3p(s) , is treated with water. formula: na_{3}p(aq) +h_{2}o(l) -> f_{3}(g) +naoh(aq) na3p(aq)+h2o(l)⟶ph3(g)+naoh(aq) write the balanced equation for the reaction of na3p(s) with h2o(l) . phases are optional. balanced equation: na_{3}p +3 h _{2}o-> ph_{3} +3naoh

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1

Chemistry, 23.06.2019 15:00
How much more basic is a solution with ph 8 than a solution with ph 7
Answers: 1
You know the right answer?
When lithium nitride, li3n(s) , is treated with water, h2o(l) , ammonia, nh3(g) , is produced. predi...
Questions

Social Studies, 03.11.2019 05:31



Mathematics, 03.11.2019 05:31


Mathematics, 03.11.2019 05:31

English, 03.11.2019 05:31

Arts, 03.11.2019 05:31

Mathematics, 03.11.2019 05:31

Social Studies, 03.11.2019 05:31

English, 03.11.2019 05:31




History, 03.11.2019 06:31

Mathematics, 03.11.2019 06:31

Mathematics, 03.11.2019 06:31



Business, 03.11.2019 06:31