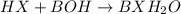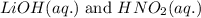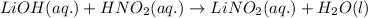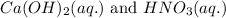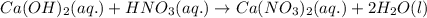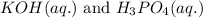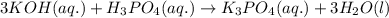Chemistry, 02.08.2019 22:20 Animallover100
Write the complete equation for the neutralization reactions that take place when the following water solutions are mixed. (if an acid has more than one acidic hydrogen, assume that there is enough base to remove all of them. assume that there is enough acid to neutralize all of the basic hydroxide ions.) a. lioh(aq) hno2(aq) b. co(oh)2(s) hno3(aq) c. h3po4(aq) koh(aq

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
Write the complete equation for the neutralization reactions that take place when the following wate...
Questions


Law, 16.01.2021 22:30






Computers and Technology, 16.01.2021 22:30

Biology, 16.01.2021 22:30



Computers and Technology, 16.01.2021 22:30




Mathematics, 16.01.2021 22:30

Mathematics, 16.01.2021 22:30

Mathematics, 16.01.2021 22:30

Health, 16.01.2021 22:30

Arts, 16.01.2021 22:40