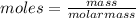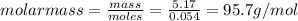Asample of an unknown compound is vaporized at 170 c. the gas produced has a volume of 1980 ml at a pressure of 1 atm, and it weighs 5.17 gr. assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. round your answer to 3 significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2


Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Asample of an unknown compound is vaporized at 170 c. the gas produced has a volume of 1980 ml at a...
Questions

Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10


History, 03.12.2020 07:10







Social Studies, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

English, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10

Mathematics, 03.12.2020 07:10


Mathematics, 03.12.2020 07:10

Chemistry, 03.12.2020 07:10