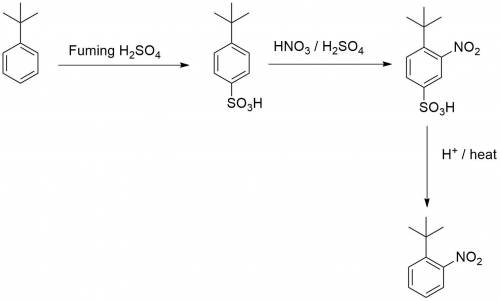
Chemistry, 02.08.2019 23:20 kkeith121p6ujlt
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you could leverage this to allow you to make just 1-(1,1-dimethylethyl)-2-nitrobenzen e from (1,1-dimethylethyl)benzene.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you...
Questions

Social Studies, 01.10.2019 22:00

English, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00






English, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00



Biology, 01.10.2019 22:00

Geography, 01.10.2019 22:00

History, 01.10.2019 22:00

Social Studies, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

Social Studies, 01.10.2019 22:00


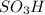 groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.
groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.