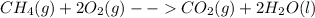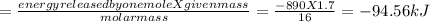Chemistry, 03.08.2019 00:20 jtorres0520
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon dioxide and water: ch4 (g) 2o2 (g) → co2 (g) 2h2o(l) δh = -890.0 kj
calculate the value of q (kj) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
(a) -94.6 kj
(b) 0.0306 kj
(c) -0.0106 kj
(d) 32.7 kj
(e) -9.46 × 10^4 kj

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon di...
Questions


Mathematics, 06.11.2020 17:30

Spanish, 06.11.2020 17:30

English, 06.11.2020 17:30


Mathematics, 06.11.2020 17:30

Physics, 06.11.2020 17:30

Social Studies, 06.11.2020 17:30

Mathematics, 06.11.2020 17:30

Mathematics, 06.11.2020 17:30

Mathematics, 06.11.2020 17:30



Mathematics, 06.11.2020 17:30





English, 06.11.2020 17:30