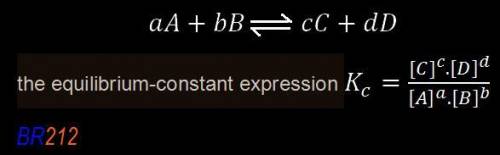Chemistry, 03.08.2019 02:30 sainijasdeep27
Write the equilibrium‑constant expression for the reaction a(s)+3b(l)↽−−⇀2c(aq)+d(aq) in terms of [a], [b], [c], and [d], as needed. note that , which is sometimes symbolized as , denotes that the equilibrium constant is expressed using molar concentrations. for this question, means the same thing as .

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1


Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
Write the equilibrium‑constant expression for the reaction a(s)+3b(l)↽−−⇀2c(aq)+d(aq) in terms of [a...
Questions

Mathematics, 09.03.2021 06:40


Mathematics, 09.03.2021 06:40



Chemistry, 09.03.2021 06:40



Mathematics, 09.03.2021 06:40

English, 09.03.2021 06:40

Spanish, 09.03.2021 06:40


Mathematics, 09.03.2021 06:40



Mathematics, 09.03.2021 06:40


Mathematics, 09.03.2021 06:40


Mathematics, 09.03.2021 06:40

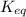
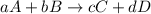
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0163/9721/9c8b0.png)
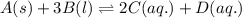
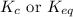 for the given reaction follows:
for the given reaction follows:![K_{c}=\frac{[C]^2[D]^1}{[A]^1[B]^3}](/tpl/images/0163/9721/f02fe.png)
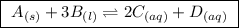 , we can observe that the substances on the right-hand side have a solution phase (aq) that is allowed into the equilibrium constant K.
, we can observe that the substances on the right-hand side have a solution phase (aq) that is allowed into the equilibrium constant K.![\boxed{ \ K = \frac{[C]^2.[D]}{[A].[B]^3} \ }](/tpl/images/0163/9721/e01b1.png) we get the final result
we get the final result ![\boxed{ \ K = [C]^2.[D] \ }](/tpl/images/0163/9721/83dc2.png)
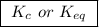 , denotes that the equilibrium constant is expressed using molar concentrations, i.e.,
, denotes that the equilibrium constant is expressed using molar concentrations, i.e., 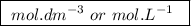 . For this question,
. For this question, 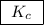 means the same thing as
means the same thing as 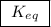 .The rIght-hand side of the equation on top, left-hand side of the equation on the bottom.The square brackets show concentrations in
.The rIght-hand side of the equation on top, left-hand side of the equation on the bottom.The square brackets show concentrations in