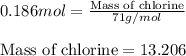Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
How many grams of cl2 (mw = 71.0 g/mol) can be prepared from the reaction of 16.0 g of mno2 (fw = 86...
Questions

English, 09.12.2019 21:31







Mathematics, 09.12.2019 21:31

Mathematics, 09.12.2019 21:31



Mathematics, 09.12.2019 21:31

Health, 09.12.2019 21:31



Chemistry, 09.12.2019 21:31



Mathematics, 09.12.2019 21:31

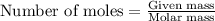 .....(1)
.....(1)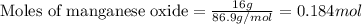
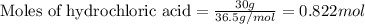
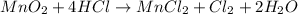
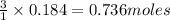 of hydrochloric acid
of hydrochloric acid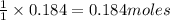 of chlorine.
of chlorine.