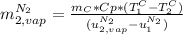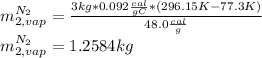Chemistry, 05.08.2019 17:10 michaelwthms
A3.00-kg block of copper at 23.0°c is dropped into a large vessel of liquid nitrogen at 77.3 k. how many kilograms of nitrogen boil away by the time the copper reaches 77.3 k? (the specific heat of copper is 0.092 0 cal/g · °c, and the latent heat of vaporization of nitrogen is 48.0 cal/g.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
A3.00-kg block of copper at 23.0°c is dropped into a large vessel of liquid nitrogen at 77.3 k. how...
Questions

History, 14.05.2021 02:30








History, 14.05.2021 02:30

Mathematics, 14.05.2021 02:30









Health, 14.05.2021 02:30

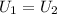
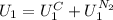
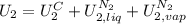
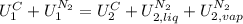
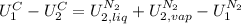
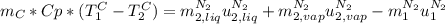
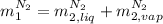 ,
,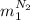 in the energy balance:
in the energy balance: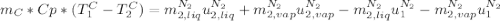
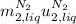 disappear because
disappear because 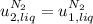 (The specific energy in the liquid is the same because the temperature does not change).
(The specific energy in the liquid is the same because the temperature does not change).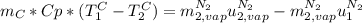
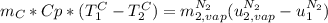
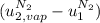 is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so:
is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so: