

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
What is the ph of an aqueous solution made by combining 35.81 ml of a 0.4364 m sodium acetate with 3...
Questions


Mathematics, 22.07.2020 20:01


Computers and Technology, 22.07.2020 20:01







Mathematics, 22.07.2020 20:01






Mathematics, 22.07.2020 20:01



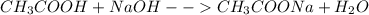
![pH=pKa+log\frac{[salt]}{[acid]}=4.74+log\frac{0.0158}{0.0119}=4.86](/tpl/images/0171/8130/95e51.png)


