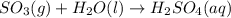Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decrease in the ph of water by increasing the h+ concentration. complete the reaction between sulfur trioxide and water in the formation of acid rain. include the phase of the acid produced.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3


Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decre...
Questions

Social Studies, 06.02.2022 03:50

History, 06.02.2022 03:50




Computers and Technology, 06.02.2022 04:00



Mathematics, 06.02.2022 04:00


Social Studies, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

Mathematics, 06.02.2022 04:00

Geography, 06.02.2022 04:00






Mathematics, 06.02.2022 04:00