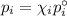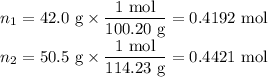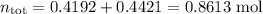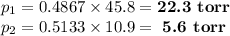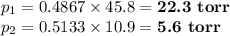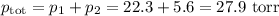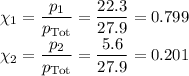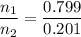Chemistry, 05.08.2019 21:20 DragonLovely
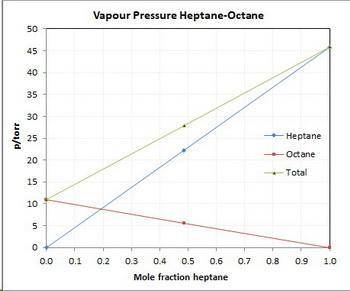
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressures of pure heptane and pure octane at 25 ∘c are 45.8 torr and 10.9 torr, respectively. assuming ideal behavior, calculate each of the following. (note that the mole fraction of an individual gas component in an ideal gas mixture can be expressed in terms of the component's partial pressure.)a.) the vapor pressure of each of the solution components in the mixtureb.) the total pressure above the solutionc.) the composition of the vapor in mass percentd.) why is the composition of the vapor different from the composition of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Asolution contains 42.0 g of heptane (c7h16) and 50.5 g of octane (c8h18) at 25 ∘c. the vapor pressu...
Questions





Mathematics, 30.03.2020 21:16

Mathematics, 30.03.2020 21:16

English, 30.03.2020 21:16

Mathematics, 30.03.2020 21:16

Mathematics, 30.03.2020 21:16


Mathematics, 30.03.2020 21:16

Mathematics, 30.03.2020 21:16








Mathematics, 30.03.2020 21:16