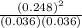Chemistry, 05.08.2019 21:20 ARAYAMYHAND
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present in a flask. the h2 and i2 react according to the balanced equation below. h2(g) + i2(g) 2 hi(g)when equilibrium is reached, the concentration of h2(g) has decreased to 0.036 mol/l. what is the equilibrium constant, kc, for the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
At a high temperature, equal concentrations of 0.160 mol/l of h2(g) and i2(g) are initially present...
Questions


Mathematics, 17.12.2020 20:40


Mathematics, 17.12.2020 20:40



History, 17.12.2020 20:40

Mathematics, 17.12.2020 20:40

Mathematics, 17.12.2020 20:40



Mathematics, 17.12.2020 20:40



History, 17.12.2020 20:40

English, 17.12.2020 20:40



Chemistry, 17.12.2020 20:40

Mathematics, 17.12.2020 20:40

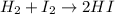
![[H_{2}]](/tpl/images/0171/8442/f6f78.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M![[I_{2}]](/tpl/images/0171/8442/d0626.png) = [0.160 - x] = 0.036 M
= [0.160 - x] = 0.036 M 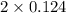
![K_{eq} = \frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0171/8442/ed373.png)