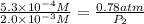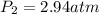Chemistry, 05.08.2019 23:30 Tyrant4life
On a clear day at sea level, with a temperature of 25 °c, the partial pressure of n2 in air is 0.78 atm and the concentration of nitrogen in water is 5.3⋅10−4 m. when the partial pressure of n2 is atm, the concentration in water is 2.0⋅10−3 m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
On a clear day at sea level, with a temperature of 25 °c, the partial pressure of n2 in air is 0.78...
Questions




Mathematics, 05.03.2020 01:42

Mathematics, 05.03.2020 01:43

Biology, 05.03.2020 01:43

Mathematics, 05.03.2020 01:43

English, 05.03.2020 01:43

English, 05.03.2020 01:43

History, 05.03.2020 01:44








Computers and Technology, 05.03.2020 01:44


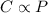
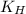 is Henry's constant.
is Henry's constant.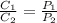
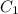 = initial concentration of gas =
= initial concentration of gas = 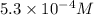
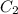 = final concentration of gas =
= final concentration of gas = 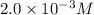
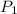 = initial partial pressure of gas = 0.78 atm
= initial partial pressure of gas = 0.78 atm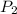 = final partial pressure of gas = ?
= final partial pressure of gas = ?