Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
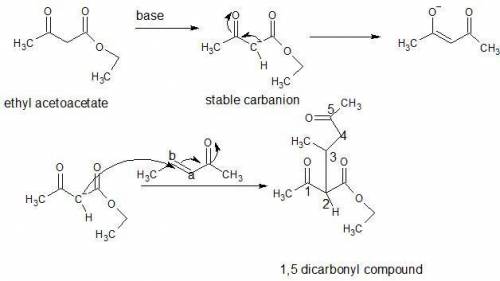
In a michael reaction, an a carbon attacks the b carbon of an a, b-unsaturated carbonyl compound to...
Questions


Mathematics, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50





Physics, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50

English, 29.10.2020 20:50




English, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50


Mathematics, 29.10.2020 20:50



History, 29.10.2020 20:50