

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
The titrate 20.00 milliliters of an unknown hc2h3o2 smaple, use 43.00 of a standard 0.1 m naoh titra...
Questions

English, 11.01.2020 21:31

Mathematics, 11.01.2020 21:31

Mathematics, 11.01.2020 21:31

Mathematics, 11.01.2020 21:31

Mathematics, 11.01.2020 21:31


Mathematics, 11.01.2020 21:31

Mathematics, 11.01.2020 21:31



Mathematics, 11.01.2020 21:31


Geography, 11.01.2020 21:31


Social Studies, 11.01.2020 21:31





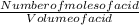
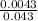 = 0.1M
= 0.1M

