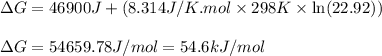Chemistry, 06.08.2019 01:10 candymorgan81
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c under these conditions: pco2pccl4pcocl2===0.140atm0.180atm0 .760atm δg∘f for co2(g) is −394.4kj/mol, δg∘f for ccl4(g) is −62.3kj/mol, and δg∘f for cocl2(g) is −204.9kj/mol. express the energy change in kilojoules per mole to one decimal place.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Consider the following reaction: co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at25 ∘c un...
Questions


Biology, 25.09.2019 02:30

Business, 25.09.2019 02:30

Mathematics, 25.09.2019 02:30






Health, 25.09.2019 02:30


Mathematics, 25.09.2019 02:30


Geography, 25.09.2019 02:30





Mathematics, 25.09.2019 02:30

Mathematics, 25.09.2019 02:30

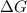 for the reaction is 54.6 kJ/mol
for the reaction is 54.6 kJ/mol 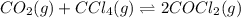
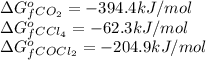
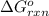 for the reaction, we use the equation:
for the reaction, we use the equation:![\Delta G^o_{rxn}=\sum [n\times \Delta G_f(product)]-\sum [n\times \Delta G_f(reactant)]](/tpl/images/0171/9686/1c133.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(COCl_2)})]-[(1\times \Delta G^o_f_{(CO_2)})+(1\times \Delta G^o_f_{(CCl_4)})]](/tpl/images/0171/9686/08d2c.png)
![\Delta G^o_{rxn}=[(2\times (-204.9))-((1\times (-394.4))+(1\times (-62.3)))]\\\Delta G^o_{rxn}=46.9kJ=46900J](/tpl/images/0171/9686/b07a7.png)
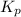 for the given reaction:
for the given reaction: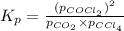
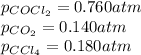
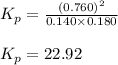
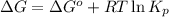
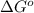 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J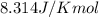
![25^oC=[25+273]K=298K](/tpl/images/0171/9686/df1f6.png)