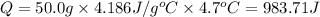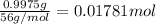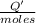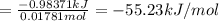Chemistry, 06.08.2019 01:20 larissacrystalow8g2w
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperature is 22.3 degrees celsius. after addition of the solid, the solution temperature is raised to about 27.0 degrees celsius. the substance is known to have a molar mass of about 56 g/mol. calculate the enthlapy of solution in kj/mol.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

You know the right answer?
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperatur...
Questions

Mathematics, 29.01.2020 09:47


History, 29.01.2020 09:47



Mathematics, 29.01.2020 09:47

History, 29.01.2020 09:47


Mathematics, 29.01.2020 09:47

History, 29.01.2020 09:47




English, 29.01.2020 09:47

Mathematics, 29.01.2020 09:47



Mathematics, 29.01.2020 09:47


Computers and Technology, 29.01.2020 09:47