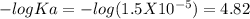Chemistry, 06.08.2019 01:30 darius12318
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solution that is 0.682 min butanoic acid (hc4h7o2) and 0.674 min butanoate ion (c4h7o2–). calculate the ph of (a) and (b) before and after the addition of the naoh. assume volumes are additive. (ka, hc4h7o2= 1.5 × 10-5)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solu...
Questions



Mathematics, 22.10.2020 14:01

History, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01


Mathematics, 22.10.2020 14:01


Mathematics, 22.10.2020 14:01



Mathematics, 22.10.2020 14:01

Computers and Technology, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01

Physics, 22.10.2020 14:01


Social Studies, 22.10.2020 14:01


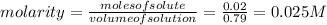
![pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0171/9988/ec35f.png)