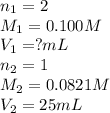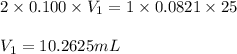Chemistry, 06.08.2019 01:30 heavendavis101
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(aq) + 2 h2o(l) calculate the volume of 0.100 m sulfuric acid required to neutralize 25.0 ml of 0.0821 m koh.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(...
Questions

History, 06.05.2020 21:57



History, 06.05.2020 21:57

Engineering, 06.05.2020 21:57

History, 06.05.2020 21:57

Health, 06.05.2020 21:57

Mathematics, 06.05.2020 21:57



Chemistry, 06.05.2020 21:57

Physics, 06.05.2020 21:57

History, 06.05.2020 21:57





Mathematics, 06.05.2020 21:57

Social Studies, 06.05.2020 21:57

Mathematics, 06.05.2020 21:57

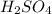 comes out to be 10.2625 mL.
comes out to be 10.2625 mL.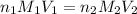
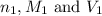 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 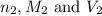 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.