
Solve for the standard entropy change (δs⁰) with each reaction below. as practice, try to predict what the sign would be before you solve it and see if it matches up.
a. n2(g) + 3 h2(g) ⇌ 2nh3(g)
b. nh4cl(s) ⇌ nh3(g) + hcl(g)
c. co(g) + 2h2(g) ⇌ ch3oh(l)
d. li3n(s) + 3h2o(l) ⇌ 3 lioh(aq) + nh3(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2



Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Solve for the standard entropy change (δs⁰) with each reaction below. as practice, try to predict wh...
Questions

History, 26.04.2021 23:30

Physics, 26.04.2021 23:30




Mathematics, 26.04.2021 23:30

Mathematics, 26.04.2021 23:30


Mathematics, 26.04.2021 23:30




Mathematics, 26.04.2021 23:30

Mathematics, 26.04.2021 23:30




Mathematics, 26.04.2021 23:30


Mathematics, 26.04.2021 23:30

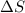 means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.
means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.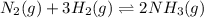 , total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of
, total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of 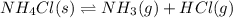 , total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of
, total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of 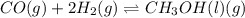 , total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of
, total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of 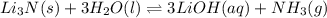 , total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of
, total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of 

