
Chemistry, 06.08.2019 02:30 jayjay9434
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the reaction starts with a mixture of pcl5, pcl3, and cl2 at pressures 0.177 atm, 0.223 atm, and 0.111 atm, respectively, at 250ºc. when the mixture comes to equilibrium at that temperature, which pressures will have decreased and which will have increased? explain why.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the re...
Questions

Physics, 04.11.2020 23:30

History, 04.11.2020 23:30

English, 04.11.2020 23:30


Arts, 04.11.2020 23:30


Biology, 04.11.2020 23:30

Mathematics, 04.11.2020 23:30

Computers and Technology, 04.11.2020 23:30

Physics, 04.11.2020 23:30



Mathematics, 04.11.2020 23:30





Mathematics, 04.11.2020 23:30


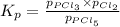
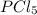 ,
, 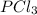 , and
, and 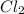 at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.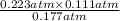
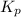 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of 

