
Chemistry, 06.08.2019 03:30 lindsaynielsen13
The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. if you were to react 93.3 g of potassium chlorate with excess red phosphorus (p4), to produce tetraphosphorus decaoxide and potassium chloride, what mass of tetraphosphorus decaoxide would be produed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1


Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a...
Questions

Mathematics, 06.09.2019 22:10

Mathematics, 06.09.2019 22:10








English, 06.09.2019 22:10



Mathematics, 06.09.2019 22:10




Mathematics, 06.09.2019 22:10

Mathematics, 06.09.2019 22:10


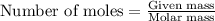 ....(1)
....(1)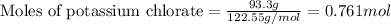
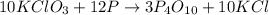
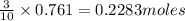 of tetraphosphorus decaoxide
of tetraphosphorus decaoxide



