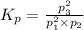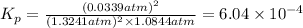Chemistry, 06.08.2019 05:10 alexsk6357
What is the value of kp for the reaction of nitrogen and oxygen to make dinitrogen monoxide if the equilibrium partial pressures of nitrogen is 1.3241 atm, the partial pressure of oxygen is 1.0844 atm and the partial pressure of dinitrogen monoxide is 0.0339 atm?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
What is the value of kp for the reaction of nitrogen and oxygen to make dinitrogen monoxide if the e...
Questions

Physics, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00

English, 06.02.2021 01:00



English, 06.02.2021 01:00



World Languages, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Biology, 06.02.2021 01:00

Arts, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00




Mathematics, 06.02.2021 01:00

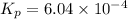
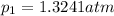
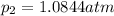
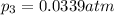
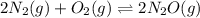
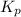 will be given as:
will be given as: