Chemistry, 06.08.2019 05:10 palomaresmitchelle
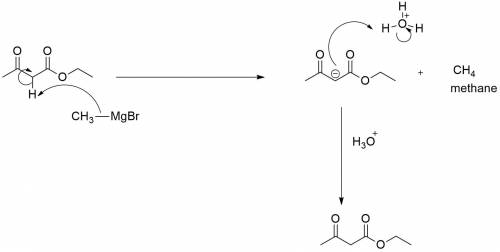
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evolved from the reaction mixture, and after adding aqueous acid, ethyl acetoacetate is recovered in high yield. identify the gas formed and explain why the starting material was recovered in this reaction. be sure to answer all parts.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evol...
Questions


English, 25.07.2020 01:01


English, 25.07.2020 01:01








Chemistry, 25.07.2020 01:01

Biology, 25.07.2020 01:01


History, 25.07.2020 01:01


Mathematics, 25.07.2020 01:01


Mathematics, 25.07.2020 01:01

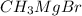 acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.
acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.