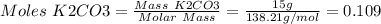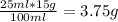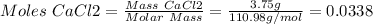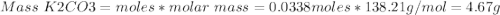Chemistry, 06.08.2019 19:30 santosbeti90
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and calcium chloride. 2. using this balanced equation, determine the limiting reactant if 15 grams of calcium chloride was reacted with 15 grams of potassium carbonate. 3. using your answer for question 2, determine the mass of potassium carbonate needed to fully precipitate all the calcium from a 25 ml sample of 15% calcium chloride.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and...
Questions

SAT, 26.11.2021 19:00


Spanish, 26.11.2021 19:00



Mathematics, 26.11.2021 19:00


Social Studies, 26.11.2021 19:00





Social Studies, 26.11.2021 19:00




Social Studies, 26.11.2021 19:00

History, 26.11.2021 19:00

Mathematics, 26.11.2021 19:00

Biology, 26.11.2021 19:00

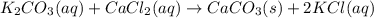
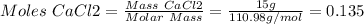
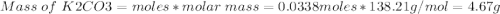 = 15 g
= 15 g