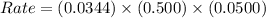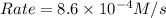Chemistry, 06.08.2019 23:20 cheyennebatz3609
The following data is given to you about a reaction you are studying: overall reaction: 2a d proposed mechanism: step 1 a + b c (slow) step 2 c + a d + b (fast) [a]o = 0.500 m [b]o = 0.0500 m [c]o = 0.500 m [d]o = 1.50 m this reaction was run at a series of temperatures and it was found that a plot of ln(k) vs 1/t (k) gives a straight line with a slope of -982.7 and a y intercept of -0.0726. what is the initial rate of the reaction at 298k?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
The following data is given to you about a reaction you are studying: overall reaction: 2a d pro...
Questions

Mathematics, 12.03.2021 04:10


Mathematics, 12.03.2021 04:10

Social Studies, 12.03.2021 04:10



Mathematics, 12.03.2021 04:10




Computers and Technology, 12.03.2021 04:10

History, 12.03.2021 04:10



Geography, 12.03.2021 04:10


Mathematics, 12.03.2021 04:10



Mathematics, 12.03.2021 04:10

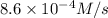
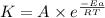
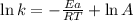 ............(1)
............(1)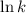 vs
vs 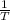 then the graph shows a straight line with negative slope. That means,
then the graph shows a straight line with negative slope. That means,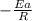

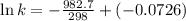
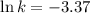

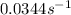
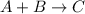
![Rate=k[A][B]](/tpl/images/0172/4346/27e48.png)