
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the following concentrations are present: [so2] = 0.010 m; [so3] = 10.m; [o2] = 0.010 m. is the mixture at equilibrium? if not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium? yes, the mixture is at equilibrium. no, left to right. no, right to left. there is not enough information to be able to predict the direction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2
You know the right answer?
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the fo...
Questions

Mathematics, 17.03.2020 21:04

Mathematics, 17.03.2020 21:04









Mathematics, 17.03.2020 21:04



History, 17.03.2020 21:04

English, 17.03.2020 21:05



Mathematics, 17.03.2020 21:05

Geography, 17.03.2020 21:05

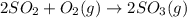
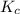 = 4.3 \times 10^{6}[/tex].
= 4.3 \times 10^{6}[/tex].![[SO_2]](/tpl/images/0172/4735/0303a.png) = 0.010 M;
= 0.010 M; ![[SO_3]](/tpl/images/0172/4735/8a06f.png) = 10.M;
= 10.M; ![[O_2]](/tpl/images/0172/4735/b0db0.png) = 0.010 M.
= 0.010 M.![\frac{[SO_{3}]^{2}}{[SO_{2}]^{2}[O_{2}]}](/tpl/images/0172/4735/2750c.png)
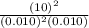
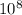
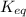 , then reaction moves in the backward direction.
, then reaction moves in the backward direction.

