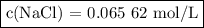Chemistry, 07.08.2019 01:10 BrainlyAvenger
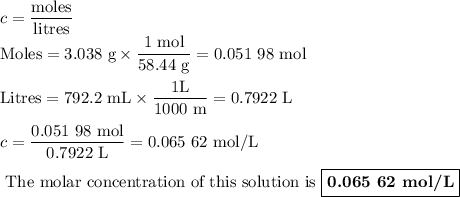
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solution of nacl by dissolving 3.038 g of nacl in enough
water to make 792.2 ml of solution. what is the molarity of this solution?
c(nacl)=

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solutio...
Questions

Mathematics, 20.06.2020 08:57

History, 20.06.2020 08:57






History, 20.06.2020 08:57




Arts, 20.06.2020 08:57


English, 20.06.2020 08:57


Mathematics, 20.06.2020 08:57



Mathematics, 20.06.2020 08:57

Mathematics, 20.06.2020 08:57