
Chemistry, 07.08.2019 03:10 Jazminnexoxo1093
The following thermodynamic data are available for octane, oxygen gas, carbon dioxide gas, water, and water vapor: molecule δh∘f (kj/mol) c8h18(l) −250.1 o2(g) 0 co2(g) −393.5 h2o(l) −285.8 h2o(g) −241.8 part b calculate δhrxn for the combustion of octane by using enthalpies of formation from the transition above. express the energy in kilojoules per mole to three significant figures. δhrxn δ h r x n = nothing kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The following thermodynamic data are available for octane, oxygen gas, carbon dioxide gas, water, an...
Questions

English, 05.07.2019 14:30

Social Studies, 05.07.2019 14:30

History, 05.07.2019 14:30

Health, 05.07.2019 14:30




Chemistry, 05.07.2019 14:30

Biology, 05.07.2019 14:30

History, 05.07.2019 14:30


Business, 05.07.2019 14:30

Mathematics, 05.07.2019 14:30

Advanced Placement (AP), 05.07.2019 14:30

Mathematics, 05.07.2019 14:30


Mathematics, 05.07.2019 14:30

Business, 05.07.2019 14:30


Mathematics, 05.07.2019 14:30

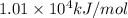
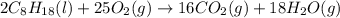
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0172/5689/45485.png)
![\Delta H^o_{rxn}=[(16\times \Delta H^o_f_{(CO_2(g))})+(18\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(C_8H_{18}(l))})+(25\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0172/5689/36b5a.png)
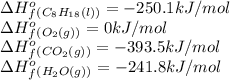
![\Delta H^o_{rxn}=[(16\times (-393.5))+(18\times (-241.8))]-[(2\times (-250.1))+(25\times (0))]=10148.2kJ/mol=1.01\times 10^4kJ/mol](/tpl/images/0172/5689/a24a9.png)


