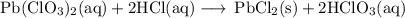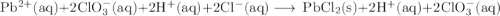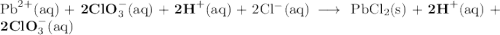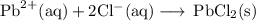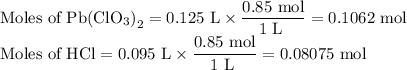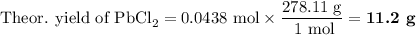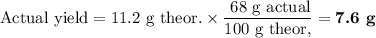Chemistry, 07.08.2019 04:10 kraigstlistt
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecular, ionic, and net equations with state labels. use solubility rules and knowledge of strong acids. predict how many grams of what solid product can be collected if 125ml lead ll chlorate was treated with with 95 ml of the hydrologic acid. if percent yield was 68 percent how much product was collected and what is the molarity of the chlorate? what is the final concentration of pb2+. show work!

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecu...
Questions


Physics, 25.03.2020 17:51

Chemistry, 25.03.2020 17:52


Spanish, 25.03.2020 17:52


English, 25.03.2020 17:52




Mathematics, 25.03.2020 17:52





Social Studies, 25.03.2020 17:52