
Chemistry, 07.08.2019 06:10 makeda2010
A125 g sample of iron (iii) bromide, febr3, is weighed out on a balance. (a) how many moles of the compound are present? (b) how many iron (iii) ions are present in the sample? (c) how many ions of bromide are present in the sample? an excellent response will clearly show each step in your reasoning, indicating the units (dimensions) of the answers and consideration of significant figures. for answers greater than 1000 or less than 0.1 scientific notation should be used.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3


Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A125 g sample of iron (iii) bromide, febr3, is weighed out on a balance. (a) how many moles of the c...
Questions





Computers and Technology, 02.01.2020 20:31

Social Studies, 02.01.2020 20:31








Computers and Technology, 02.01.2020 20:31

Social Studies, 02.01.2020 20:31


Social Studies, 02.01.2020 20:31

Biology, 02.01.2020 20:31


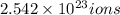 of iron(III)
of iron(III)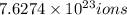 of bromide.
of bromide.
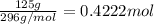
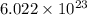 molecules
molecules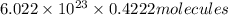
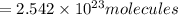 of iron (III) bromide
of iron (III) bromide 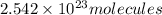 will contain:
will contain: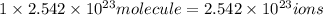 of iron(III)
of iron(III)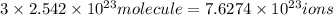 of bromide.
of bromide.

