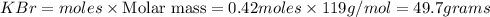Chemistry, 07.08.2019 17:10 risolatziyovudd
Calculate how many grams of the product form when 16.7 g of liquid bromine reacts with solid potassium. assume that there is more than enough of the solid potassium. 2 k(s) + br2(l) → 2 kbr(s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Calculate how many grams of the product form when 16.7 g of liquid bromine reacts with solid potassi...
Questions

Mathematics, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00



English, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00



Mathematics, 29.03.2021 23:00




Mathematics, 29.03.2021 23:00



Mathematics, 29.03.2021 23:00


History, 29.03.2021 23:00

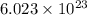 of particles.
of particles.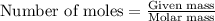
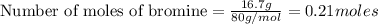
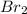 acts as limiting reagent as it limits the formation of product as potassium is in excess.
acts as limiting reagent as it limits the formation of product as potassium is in excess.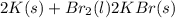
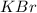
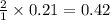 moles of
moles of