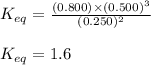Chemistry, 07.08.2019 17:20 kellymcdow9385
For the reaction 2nh3(g)↽−−⇀ 3h2(g)+n2(g) the equilibrium concentrations were found to be [nh3]=0.250 m , [h2]=0.500 m , and [n2]=0.800 m . what is the equilibrium constant for this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
For the reaction 2nh3(g)↽−−⇀ 3h2(g)+n2(g) the equilibrium concentrations were found to be [nh3]=0.25...
Questions

Mathematics, 11.08.2021 04:50

Chemistry, 11.08.2021 04:50




Mathematics, 11.08.2021 04:50

Mathematics, 11.08.2021 04:50

Mathematics, 11.08.2021 04:50




Mathematics, 11.08.2021 04:50

Biology, 11.08.2021 04:50

Mathematics, 11.08.2021 04:50


Health, 11.08.2021 04:50


Mathematics, 11.08.2021 04:50



![K_{eq}=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0172/7166/804f3.png)
![[NH_3]=0.250M](/tpl/images/0172/7166/0d43e.png)
![[H_2]=0.500M](/tpl/images/0172/7166/5e4b7.png)
![[N_2]=0.800M](/tpl/images/0172/7166/3aba5.png)