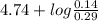Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Calculate the ph after 10 ml of 1.0 m sodium hydroxide is added to 60 ml of 0.5m acetic acid....
Questions

Mathematics, 03.02.2021 01:00


English, 03.02.2021 01:00


Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Computers and Technology, 03.02.2021 01:00

Spanish, 03.02.2021 01:00








Mathematics, 03.02.2021 01:00

Physics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

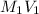 =
= 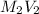
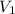 = total volume
= total volume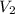 = initial volume
= initial volume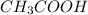 =
= 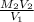
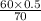
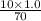
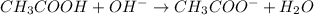
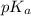 = 4.74
= 4.74![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]}](/tpl/images/0173/1451/85431.png)