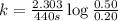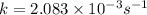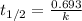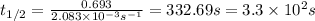Chemistry, 08.08.2019 05:30 billy583887
What is the half-life of a first-order reaction if it takes 4.4 x 102 seconds for the concentration to decrease from 0.50 m to 0.20 m? 2.5 x 102 s 3.3 x 102s 1.6s 21 s 27 s

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
What is the half-life of a first-order reaction if it takes 4.4 x 102 seconds for the concentration...
Questions




Mathematics, 01.10.2019 18:30

History, 01.10.2019 18:30

Chemistry, 01.10.2019 18:30

Mathematics, 01.10.2019 18:30

Chemistry, 01.10.2019 18:30


English, 01.10.2019 18:30



English, 01.10.2019 18:30

Mathematics, 01.10.2019 18:30





Physics, 01.10.2019 18:30

Mathematics, 01.10.2019 18:30

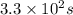
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0173/1549/f1041.png)
![[A_o]](/tpl/images/0173/1549/dc622.png) = initial amount of the reactant = 0.50 M
= initial amount of the reactant = 0.50 M