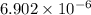Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
A2.35l solution contains 2.20 mol of weak acid hx. if 1.15 mol naoh is added to this solution, the p...
Questions


English, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01

Health, 28.09.2020 05:01

Biology, 28.09.2020 05:01

Biology, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01


Business, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01




Biology, 28.09.2020 05:01



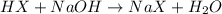
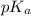 will be as follows.
will be as follows.![pK_{a} + \frac{log[NaX]}{[HX]}](/tpl/images/0173/1558/694ef.png)
![pK_{a} = pH - log \frac{log[NaX]}{[HX]}](/tpl/images/0173/1558/95a26.png)
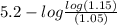
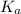 =
=