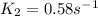Chemistry, 08.08.2019 05:30 sparky1234
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reaction is 4.60 x 10-6 s-1 at 275 k, what is the rate constant at 366 k? 0.58 1/s

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reactio...
Questions



History, 22.08.2019 01:30

History, 22.08.2019 01:30


Biology, 22.08.2019 01:30

Biology, 22.08.2019 01:30


Biology, 22.08.2019 01:30

Physics, 22.08.2019 01:30

Mathematics, 22.08.2019 01:30

Biology, 22.08.2019 01:30

History, 22.08.2019 01:30

History, 22.08.2019 01:30


Mathematics, 22.08.2019 01:30


Social Studies, 22.08.2019 01:30

Mathematics, 22.08.2019 01:30

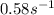
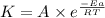
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0173/1540/6d953.png)
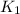 =rate constant at
=rate constant at 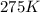 =
= 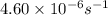
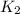 = rate constant at
= rate constant at 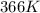 = ?
= ?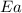 = activation energy for the reaction = 108kJ/mol=108000 J/mol
= activation energy for the reaction = 108kJ/mol=108000 J/mol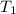 = initial temperature =
= initial temperature = 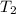 = final temperature =
= final temperature = ![\log (\frac{K_2}{4.60\times 10^{-6}})=\frac{108000}{2.303\times 8.314J/mole.K}[\frac{1}{275K}-\frac{1}{366K}]](/tpl/images/0173/1540/60a14.png)