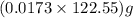8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g) + 2kcio, (s) the oxygen was collected by the displacement of water at 22'c at a total pressure of 754 torr. the volume of the gas collected was 0.65l and the vapor pressure of water at 22°c is 21 torr. calculate a) the partial pressure of o2 in the gas collected and b) the mass of kcio3 in the sample that was decomposed

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

You know the right answer?
8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g)...
Questions


English, 29.11.2020 08:20

Social Studies, 29.11.2020 08:20

Mathematics, 29.11.2020 08:20


Mathematics, 29.11.2020 08:20

Biology, 29.11.2020 08:30

Mathematics, 29.11.2020 08:30






Health, 29.11.2020 08:30



Computers and Technology, 29.11.2020 08:30

Advanced Placement (AP), 29.11.2020 08:30

Mathematics, 29.11.2020 08:30

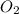 in the gas was 733 torr and mass of
in the gas was 733 torr and mass of 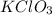 in the sample was 2.12 g.
in the sample was 2.12 g.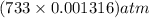 = 0.9646 atm
= 0.9646 atm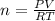
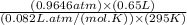
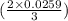 moles or 0.0173 moles of
moles or 0.0173 moles of