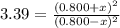At a particular temperature, k 3.39 for the reaction so2(9) + no2(9) sos(9) +no(9) if all four gases had initial concentrations of 0.800 m, calculate the equilibrium concentrations of the gases. equilibrium concentration of so2-m equilibrium concentration of no2m equilibrium concentration of so3 equilibrium concentration of no

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
At a particular temperature, k 3.39 for the reaction so2(9) + no2(9) sos(9) +no(9) if all four gases...
Questions

Social Studies, 20.09.2019 10:20



English, 20.09.2019 10:20

English, 20.09.2019 10:20

History, 20.09.2019 10:20

History, 20.09.2019 10:20

English, 20.09.2019 10:20

History, 20.09.2019 10:20


Biology, 20.09.2019 10:20


Spanish, 20.09.2019 10:20


Biology, 20.09.2019 10:20




English, 20.09.2019 10:20

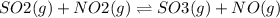
![Keq = \frac{[SO3][NO]}{[SO2][NO2]}=\frac{(0.800+x)^{2}}{(0.800-x)^{2}}](/tpl/images/0173/1735/ba675.png)