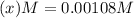Chemistry, 08.08.2019 06:20 baeethtsadia
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2 is initially placed into a 5.0-l vessel, calculate the equilibrium concentrations of all species. co2] co] o2l [02]-

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
At a particular temperature, k 5.0 x 10-6 for the reaction 2co2(9) 2co(9) +02(9) if 3.0 moles of co2...
Questions

History, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00


Mathematics, 05.10.2019 05:00

Social Studies, 05.10.2019 05:00


Mathematics, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00




History, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00

Business, 05.10.2019 05:00


Mathematics, 05.10.2019 05:00



Mathematics, 05.10.2019 05:00

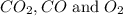 at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.
at equilibrium will be, 0.598 M, 0.00216 M and 0.00108 M respectively.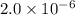
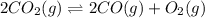
![K_c=\frac{[CO]^2[O_2]}{[CO_2]^2}](/tpl/images/0173/1740/96a03.png)
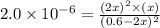
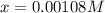
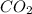 at equilibrium =
at equilibrium = ![(0.6-2x)M=[0.6-2(0.00108)]M=0.598M](/tpl/images/0173/1740/7a4da.png)
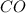 at equilibrium =
at equilibrium = ![(2x)M=[2(0.00108)]M=0.00216M](/tpl/images/0173/1740/eff4f.png)
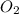 at equilibrium =
at equilibrium =