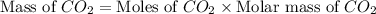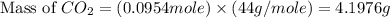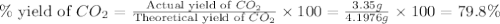Chemistry, 08.08.2019 06:20 redhot12352
Consider the reaction of c3h8 with o2 to form co2 and h2o. if 5.11 g o2 is reacted with excess c3h8 and 3.35 g of co2 is ultimately isolated, what is the percent yield for the reaction? percent yield = %

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Consider the reaction of c3h8 with o2 to form co2 and h2o. if 5.11 g o2 is reacted with excess c3h8...
Questions



Biology, 14.11.2021 20:30


Mathematics, 14.11.2021 20:30






Computers and Technology, 14.11.2021 20:30


Social Studies, 14.11.2021 20:30

Computers and Technology, 14.11.2021 20:30



Computers and Technology, 14.11.2021 20:30

English, 14.11.2021 20:30

Advanced Placement (AP), 14.11.2021 20:30

Mathematics, 14.11.2021 20:30

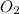 = 5.11 g
= 5.11 g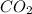 = 44 g/mole
= 44 g/mole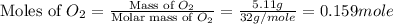
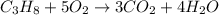
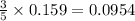 moles of
moles of